PRoVENT 2+

Update on the practice of mechanical ventilation in non-ARDS ICU patients – An international, multi-centre, prospective, observational ICU cohort study of the PROVEnet (PRoVENT 2+)
Chief Investigator
Martin Scharffenberg, MD (University Hospital Carl Gustav Carus at TU Dresden, Dresden, Germany)
Senior Investigator
Marcelo Gama de Abreu, MD, MSc, PhD, DESAIC (University Hospital Carl Gustav Carus at TU Dresden, Dresden, Germany; Cleveland Clinic, Cleveland, OH, USA)
Background and Rationale
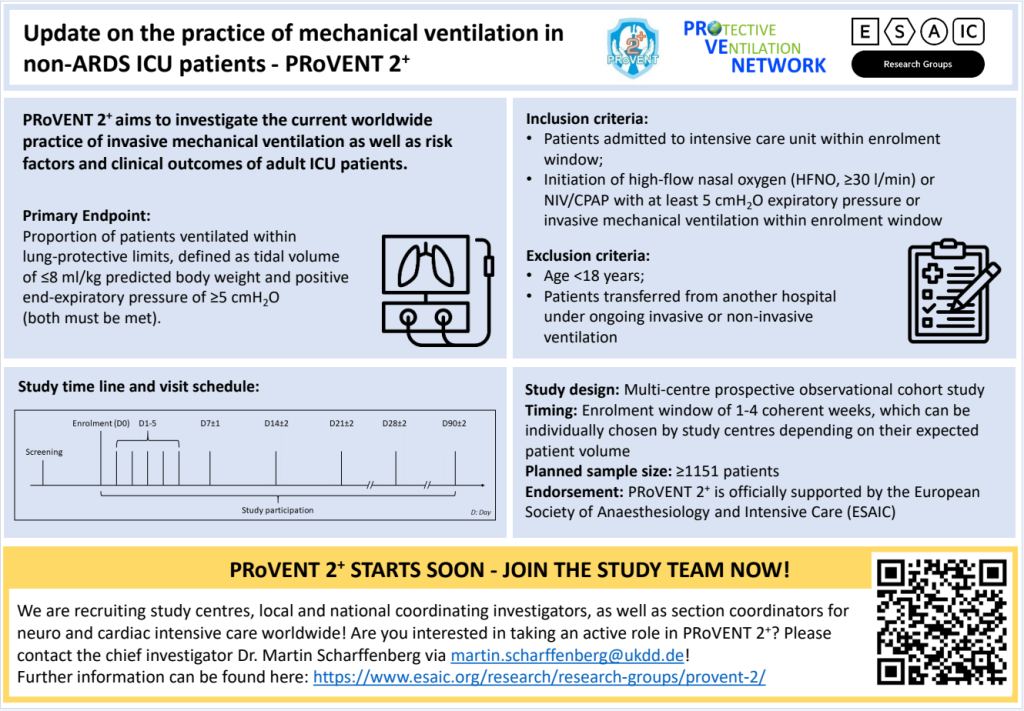
Although representing an important and often life-saving therapy, mechanical ventilation can result in ventilator-induced lung injury, distal organ damage and even death. Evidence and information about the current practice of invasive mechanical ventilation in ICU patients, especially in those not fulfilling the definition of ARDS, remains limited. Since the last large observational studies, evidence has grown, clinical procedures and routine may have changed, and awareness for lung-protective ventilation to prevent ventilator-induced lung injury may have risen over the last years. Nevertheless, clinical practice may still be sub-optimal and differ from guidelines and evidence, while geo-economical variations and even sex-related differences may still exist. Thus, this study aims to investigate the current worldwide practice of invasive mechanical ventilation as well as risk factors and clinical outcomes of adult ICU patients.
Do you want to know more about the study ?
Download the PRoVENT2+ Protocol here
Study Design
International, multi-centre, prospective, observational cohort study
Study Objectives
In invasively ventilated adult ICU patients, we aim to:
- Characterise the current practice of invasive mechanical ventilation in adult non-ARDS ICU patients (Primary objective)
Secondary objectives include:
- To describe the epidemiologic characteristics of this cohort
- To determine the proportion of patients at risk of ARDS;
- To determine the rate of progression to ARDS;
- To determine the rate of missed ARDS diagnosis;
- To determine rate and types of complications;
- To determine the mortality rate;
- To identify risk factors associated with outcome;
- To identify potentially modifiable risk factors associated with outcome;
- To identify geo–economic variations;
- To identify and compare ventilation practices reported in previous studies and in different phenotypes/sub-cohorts (e.g., neuro-surgery, thoracic surgery, COVID-19)
- To assess adjunctive diagnostics and therapies
Primary Endpoint
The following primary endpoint will be assessed: Proportion of patients ventilated within lung-protective limits, defined as tidal volume of ≤8 ml/kg predicted body weight and positive end-expiratory pressure of ≥5 cmH2O (both must be met).
Target Patient Cohort
Inclusion Criteria:
- Patients admitted to intensive care unit within the defined enrolment window
- Initiation of invasive mechanical ventilation within the enrolment window
Exclusion Criteria:
- Age <18 years
- Ventilated patients transferred from another hospital under ongoing ventilation
Sample size calculation
A sample size calculation was performed based on of data from a previous observational study on invasively ventilated non-ARDS patients. This calculation revealed a minimal sample size of 1151 patients.
Enrolment window
Study centres are allowed to choose an enrolment window of 1-4 weeks depending on their expected patient volume. The enrolment window must be a coherent period of 1-4 weeks. The timing of this window, i.e. the time point of starting the enrolment, is freely chosen by the study centre, but must be determined in advance and adhered to. Thus, participating centres must select the duration and the timing of their enrolment window and report this to the PI before starting enrolment.
Timeline
- EC approval
- Preparation of the study data base
- Call for centres: Will be announced soon
- Recruitment of patients: Will be announced soon
Join the study
PRoVENT 2+ is a prospective, international, multi-centre, observational cross-sectional study on intensive care units to mainly assess current MV strategies and outcomes in consecutive patients included in a fixed time frame. In order to achieve a representative study cohort, we aim to screen and enroll patients in hospitals worldwide. By this, PRoVENT 2+ shall be one of the largest prospective observational studies in ventilated ICU patients, where “plus” states for groups that have not included before, e.g. cardiothoracic surgery, neurosurgery and COVID-19. This can only be achieved through motivated and highly qualified physicians and scientists and close, respectful cooperation. Come on board and become a study center or investigator in the PRoVENT 2+ study. Feel free to contact us!
National Coordinating Investigators
We are looking for National Coordinating Investigators to contribute to the PRoVENT 2+ study. National Coordinating Investigators will be responsible for acquiring participating centres in their respective countries. Physicians at each site will be recruited as lead site investigators, and will provide scientific and structural leadership locally, respond for data validity and integrity, ensure compliance with local ethical and regulatory requirements, and train and monitor their local research group. Investigators are encouraged to submit and perform substudies, which are related and beneficial to the main study goal. Interested? Contact the Chief Investigator!
Steering Committee
- Martin Scharffenberg, University Hospital Carl Gustav Carus at Technische Universität Dresden, Germany
- Marcelo Gama de Abreu, Cleveland Clinic, Cleveland, Ohio, USA
- Jakob Wittenstein, University Hospital Carl Gustav Carus at Technische Universität Dresden, Germany
- Robert Huhle, University Hospital Carl Gustav Carus at Technische Universität Dresden, Germany
- Andrea Kurz, University of Graz, Austria
- Marcus Schultz, UMC, University of Amsterdam, The Netherlands
- Sabrine Hemmes, het Antoni van Leeuwenhoek, The Netherlands Cancer Institute, Amsterdam, The Netherlands
- Lorenzo Ball, University of Genova, Genova, Italy; Policlinico San Martino Hospital, Genova, Italy
- Ary Serpa Neto, Australian and New Zealand Intensive Care Research Centre (ANZIC-RC), School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia; Department of Intensive Care, Austin Hospital, Melbourne, Australia; Department of Critical Care, University of Melbourne, Melbourne, Australia; Hospital Israelita Albert Einstein, São Paulo, Brazil
- Patricia R. M. Rocco, Laboratory of Pulmonary Investigation, Carlos Chagas Filho Institute of Biophysics, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- Luigi Pisani, Mahidol Oxford Tropical Research Unit, Bangkok, Thailand & Miulli Regional Hospital, Department of Anesthesia and Intensive Care, Acquaviva delle Fonti, Italy
- Frederique Paulus, Amsterdam UMC location University of Amsterdam, Department of Intensive Care, The Netherlands; Amsterdam University of Applied Sciences, faculty of Health, Center of Expertise Urban Vitality, Amsterdam, the Netherlands
- Christian Putensen, Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany
- Paolo Pelosi†, Department of Surgical Sciences and Integrated Diagnostics, University of Genoa, Genoa, Italy; Anesthesia and Critical Care, San Martino Policlinico Hospital, IRCCS for Oncology and Neurosciences, Genoa, Italy
Publication principles
Manuscripts are going to be published under the study group name. All active contributors and collaborators are going to be acknowledged properly. We are dedicated to transparent and fair rules for co-authorship.
Financial support
The PRoVENT 2+ study is officially supported by the European Society of Anaesthesiology and Intensive Care (ESAIC) as an official ESAIC Research Group (Head: Dr. M Scharffenberg).
Contact
Dr. M. Scharffenberg, University Hospital Carl Gustav Carus at TU Dresden, Dresden, Germany; martin.scharffenberg[at]ukdd.de






