Newsletter 2022
Newsletter October 2022: Pain perception is more than just intensity
Juliane Sachau1, Manon Sendel1, Marie Péchard2, Kathrin Schnabel3, Iris Schmieg3, Terkia Medkour2, Laurent Ecochard4, Markus Woischnik5, Hiltrud Liedgens6, Esther Pogatzki-Zahn3, Didier Bouhassira2, Ralf Baron1
1Division of Neurological Pain Research and Therapy, Department of Neurology, University Hospital Schleswig-Holstein Campus Kiel, Kiel, Germany
2Inserm U987, APHP, UVSQ, Paris-Saclay University, CHU Ambroise Paré, 92100 Boulogne-Billancourt, France
3Department of Anaesthesiology, Intensive Care and Pain Medicine, University Hospital of Muenster, Münster
4Novartis Pharma AG, Basel, Switzerland
5Bayer AG, Berlin, Germany
6Grünenthal, Germany GmbH, Aachen, Germany
Corresponding author:
Dr. med. Juliane Sachau
juliane.sachau@uksh.de
“Please rate your pain on a scale ranging from ‘0’ representing ‘no pain’ to ‘10’ representing ‘pain as bad as you can imagine’.” – Ratings like this are the most common way to capture pain and treatment efficacy in current clinical trials. However, this kind of pain assessment is often inadequate and one-dimensional. This fact becomes clear when focusing on neuropathic pain.
Neuropathic pain is defined as pain that arises as a direct consequence of a lesion or disease affecting the peripheral or central somatosensory nervous system. Its prevalence in the general population is estimated at 7 to 8%, making neuropathic pain a relevant health care problem 1. Positive and negative sensory symptoms typically co-occur as a direct consequence of the nerve fiber lesion leading to a heterogenous clinical picture with both hypersensitivity and hyposensitivity to painful and non-painful stimuli. These symptoms may be accompanied by other phenomena such as a reduced functionality, sleep disturbances and psychiatric comorbidities, resulting in a significant overall burden for affected patients. Effective pain management is therefore of utmost importance to improve the quality of life of these patients. However, less than 50% of them achieve partial pain relief with the currently recommended first-line therapeutics 2. In addition, clinical trials on encouraging new drugs have failed in the past. As a result, a large proportion of patients do not currently receive adequate pain therapy. One possible reason for this therapeutic dilemma is insufficient reflection of the patient perspective in current clinical trials, which, as in the example above, focus on pain intensity as the primary outcome parameter in the vast majority of cases. However, it is quite conceivable that other outcome parameters such as the improvement in walking ability are much more important than pain intensity, at least for some of the patients. In addition, the heterogeneous selection of secondary endpoints and the use of different questionnaires leads to the fact that the results of current studies are little or not at all comparable.
So how can the treatment of chronic pain patients in general and neuropathic pain patients in particular be improved in the future? Answering this question is the aim of the Innovative Medicines Initiative (IMI) PainCare European project (www.imi.europa.eu; www.imi-paincare.eu). The IMI subtopic PROMPT focus on patient-reported outcome parameters (PROMs). The idea behind this is, that only the patients themselves can describe their pain experiences in an adequate manner. In order to capture the totality of complaints of patients with chronic pain in general and neuropathic pain in particular, various expert groups (Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) 3,4, Special Interest Group on Neuropathic Pain (NeuPSIG) 5, European Federation of Neurological Societies (EFNS) 6) have recommended a first set of outcome domains and measures, that should be assessed in clinical trials. In view of the currently unsatisfactory study situation, however, it must be questioned to what extent these recommendations are actually implemented.
As a first step towards harmonisation of outcome assessment in neuropathic pain, we therefore performed a systematic literature review (SLR) on PROMs that have been used in previous randomised controlled trials (RCTs). The aim was to identify domains and PROMs that were mainly used especially in studies with higher methodological quality.
In total, 2797 articles were identified by searching electronic databases (MEDLINE, CENTRAL, Embase) and reviewing reference lists of published meta-analyses 2,7. 251 studies met the inclusion criteria, i.e., peer-reviewed publications of RCTs assessing the efficacy of systemic or topical agents for chronic neuropathic pain (duration ≥ 3 months), and were included in the analysis. Alle used PROMs were extracted from the included studies and assigned to the previously recommended IMMPACT 4 and NeuPSIG 5 core outcome domains: ‘pain’ including ‘pain intensity’ and ‘pain other aspects’, ‘physical functioning’, ‘emotional functioning’, ‘participant ratings of global improvement and satisfaction with treatment’, ‘participant disposition’ and ‘symptoms and adverse events’. In addition, several sub-domains were defined for ‘pain other aspects’ and ‘physical functioning’ to cover the full range of their different aspects. Domains and PROMs were compared regarding the methodological quality of the studies based on the GRADE risk of bias assessment 8 (high/moderate quality n=217 versus low quality n=34) and the publication year (before n=146 versus after n=105 publication of IMMPACT/NeuPSIG recommendations).
A large amount of 200 different PROMs were used in the included studies, of which only 27 PROMs were recommended explicitly by IMMPACT and/or NeuPSIG.
At the domain level ‘pain intensity’ was – unsurprisingly – assessed by nearly all studies, regardless of the methodological study quality (high/moderate quality: 98.6%, low quality: 97.1%) (Figure 1A). The other domains were covered less frequently overall, although there were some significant differences between the study groups examined. In particular, the (sub-) domains ‘physical functioning’, ‘global improvement and satisfaction’ and ‘neuropathic pain quality’ were assessed more frequently in high/moderate quality studies and those published after 2011. However, even in good quality studies, certain (sub-) domains like ‘emotional functioning’, ‘sleep’, all subdomains of ‘pain other aspects’ and ‘participant disposition’ were rarely considered, i.e., in less than 50% of the studies.
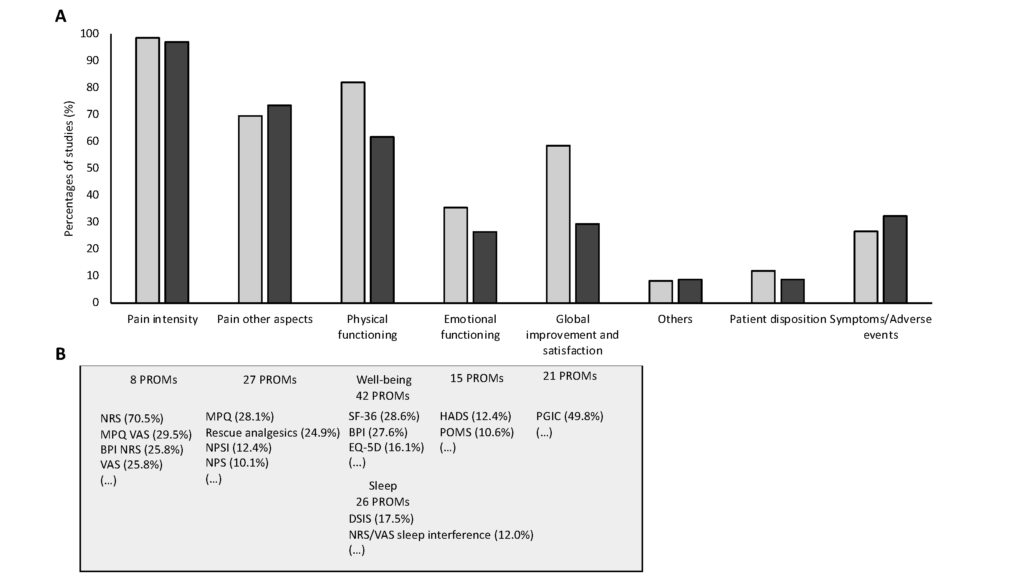
Figure A and B
At the PROM level, the assessment was also very heterogeneous in some cases, even in studies with higher quality (Figure 1B). While only eight different PROMs were used for ‘pain intensity’, ‘daily activities/well-being’ was assessed by 42 different questionnaires. Regarding the domains ‘sleep’ and ‘emotional functioning’, less than 20% of all studies used the same PROM. In addition, for some domains unknown questionnaires, i.e., questionnaires that are not described in the literature, or single questions were used.
Overall, the results of our SLR indicate that the usage of outcome parameters in neuropathic pain RCTs has increased over the last years and, in particular, in studies with better quality. This may be related to the publication of experts’ recommendations (IMMPACT, NeuPSIG). However, there is still a large heterogeneity in outcome assessment even in studies with good methodological quality, leading to a clear underrepresentation of certain domains and an insufficient comparability of study results due to the use of different PROMs. To achieve a better comparability of clinical trials and to improve treatment of patients with chronic neuropathic pain in future, the definition of a standardised core set of outcome domains and PROMs relevant to both stakeholders and patients is desperately needed.

Disclosures
This project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No [777500]. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. www.imi-paincare.eu; www.imi.europa.eu
The statements and opinions presented here reflect the author’s view and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained therein.
References
- Bouhassira D. Neuropathic pain: Definition, assessment and epidemiology. Revue Neurologique 2019; 175:16–25.
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. The Lancet. Neurology 2015; 14:162–173.
- Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19.
- Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 2003; 106:337–345.
- Haanpaa M, Attal N, Backonja M, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011; 152:14–27.
- Cruccu G, Sommer C, Anand P, et al. EFNS guidelines on neuropathic pain assessment: revised 2009. European journal of neurology 2010; 17:1010–1018.
- Moisset X, Bouhassira D, Avez Couturier J, et al. Pharmacological and non-pharmacological treatments for neuropathic pain: Systematic review and French recommendations. Revue Neurologique 2020.
- Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). Journal of Clinical Epidemiology 2011; 64:407–415.
Legends to the Figure
Figure 1. Results at the domain (A) and PROM level (B). A: Percentages of studies assessing the different IMMPACT/NeuPSIG core outcome domains by at least one PROM depending on their methodological quality (high or moderate quality (light grey) n=217, low quality (dark grey) n=34). B: The most frequently used PROMs per domain in high/moderate quality studies
NRS, Numerical Rating Scale; MPQ, McGill Pain Questionnaire; BPI, Brief Pain Inventory; VAS, Visual Analogue Scale; NPSI, Neuropathic Pain Symptom Inventory, NPS; Neuropathic Pain Scale; SF-36, 36-Item Short Form Survey; EQ-5D, Euroqol EQ-5D; DSIS, Daily Sleep Interference Scale; HADS, Hospital Anxiety and Depression Scale; POMS, Profile of Mood States; PGIC, Patient Global Impression of Change
[maxbutton id=”1″ url=”https://www.esaic.org/newsletter/” text=”Read the Newsletter” ]